Background
Due to their distinct biochemical and therapeutic characteristics, peptides have been developed as drug candidates to disrupt protein–protein interactions and target intracellular molecules such as receptor tyrosine kinases. These have turned peptide therapeutics into a leading industry with nearly 20 new peptide-based clinical trials annually, with more than 400 peptide drugs that are under global clinical developments with over 60 already approved for clinical. The increasing interest of the pharmaceutical industry in peptide therapeutics has driven various regulations in this area.
Challenge
The immunogenicity of peptide products can have clinical consequences, including production of antibodies working against the therapeutic peptide, loss of peptide efficacy, neutralization of the human peptide counterpart, and general effects impacting the immune system (including allergy and anaphylaxis). Unlike the small molecules that are superior in binding to deep folding pockets of proteins, the peptide-protein complex commonly represents larger, flat and hydrophobic binding interfaces. This leads to a complex challenge in understanding the molecular recognition mechanism and delineating binding affinity for peptide-protein interactions for both computational biologists and protein biochemists, which may further lead to limit predication in drug tolerance for the immunogenicity assessments.
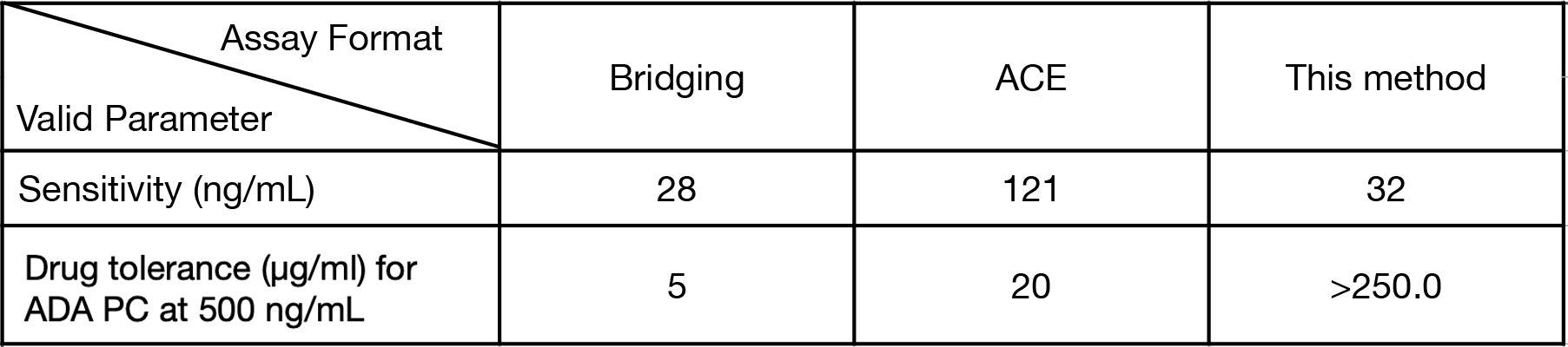
A special request with expedited timelines from a large biopharmaceutical company was received by Triangle Lab’s, regarding a pre-clinical immunogenicity study for a peptide product (MW is ca. 5kd). Prior attempts to develop an ADA assay resulted in ADA using bridging assay resulted in a sensitivity of 28 ng/mL, and a drug tolerance of 5.0 µg/mL of peptide for ADA positive control level at 500 ng/mL. A second attempt with switching to an acid capture elution (ACE) method led to a sensitivity at 121 ng/mL, and a drug tolerance at 20 µg/mL, respectively (Table 1). Those results were insufficient to meet sponsors requirements.
Table 1. Comparison of valid results among three methods.
Solution
Based on the deep expertise in traditional immunoassay platforms with a full understating of their limitations, and considerable experience with antibody enrichment from Triangle Lab scientist team, a novel method was developed involving optimization of multiple parameters. The novelty includes utilizing a recombinant fusion protein as a versatile capture antibody and preparation of a peptide drug based detection reagent using a unique peptide conjugation strategy. The developed stepwise Electrochemilumenescence Immunoassay (Scheme 1) resulted in a sensitivity of 32 ng/mL and a drug tolerance of 250 µg/mL for 500 ng/mL ADA PC. This performance meets the required levels of drug tolerance and sensitivity specific for this study. The assay was immediately validated for use to analyze pre-clinical samples within 3 weeks.
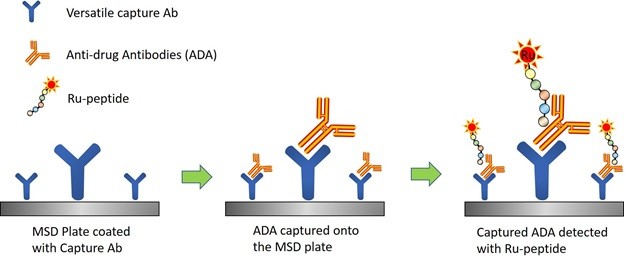
Scheme 1. Schematic illustration of the developed novel method for ADA detection for peptide drugs.
This novel method for ADA assays for peptide drugs can also be applied in other projects, such as nucleotides. Recently, the same assay format was applied to a project involving a synthetic nucleotide analog. Once again, sensitivity can be reached at 100 ng/mL with ultimate drug tolerance as high as 300 µg/mL of drug, in comparison with other assay formats which cannot be achieved. It should be noted that this novel method does not involve acid treatment to sample which is very common in traditional immunoassays, providing an additional benefit to acid-labile drug candidates.
