NAb Testing
What is NAb?

Advanced Platforms Supporting Nab

Our Experience on Nab

Regulatory Guidance
What is NAb?
Neutralization antibody is specific for antibodies that bind to the therapeutic drug and hinder its biological function. Neutralization antibody’s safety and efficacy might range from none, affecting PK/PD profile, to adverse immune response. Neutralizing antibody characterization is a subsequent and crucial step in addressing immunogenicity and the drug neutralization aspect. Unlike ADA assay, NAb assay is to detect the subset of the binding antibodies. Therefore, the drug mechanism of action plays a key role in studying these neutralizing antibodies.
Advanced Platforms Supporting Nab
More analytical technologies with high accuracy and sensitivity can be used in large molecules, biological therapeutics and ligand binding assay. We will provide solution to help you find the best analytical platform not only for your project in early or late stage of drug discovery but also in the preclinical and clinical phase. The followed platforms listed below have been successfully proven to evaluate the presence of neutralizing antibodies in the preclinical and clinical studies.

MESO SECTOR

xMark™ Microplate Absorbance Spectrophotometer

SpectraMax M5e Multi-Mode Microplate Readers
Our Experience on NAb
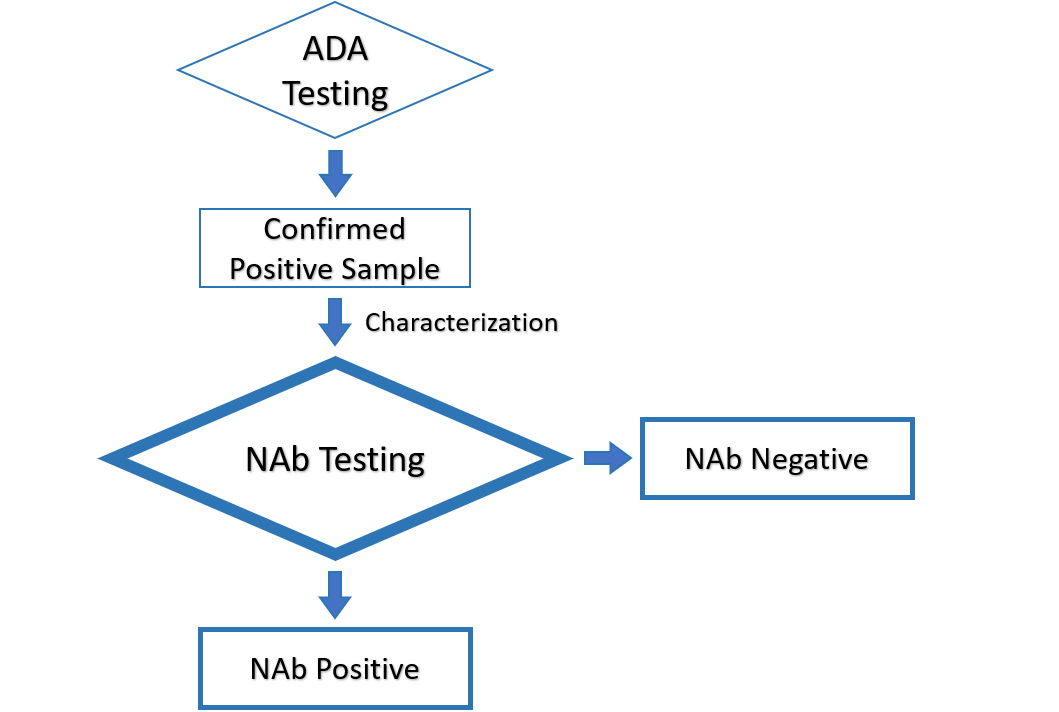
We studied drug modalities and mechanisms of action to determine whether cell-based or non cell-based assay can better reflect the NAb blocking effect on the drug functionality.
With our built-in antibody conjugation and characterization capabilities to generate labeled high affinity and specificity anti-idiotypic antibodies with consistent lot-to-lot performance, we can deliver high sensitivity Nab assays with excellent drug tolerance and consistency.
Our AI assisted assay development system is also able to guide our research for personalized and customized purposes. The machine learning enabled buffer selection software can also select the buffers based on the assay types, drug modality, assay platform, and matrixes.
Regulatory Guidance

ADA Testing of Therapeutic Protein Products – Developing and Validating Assays for Anti-Drug Antibody Detection

